Will general antiviral protocols always be science fiction?
Author: Rick Sheridan
Author: Rick Sheridan1
Date: December, 2022
Text: PDF (https://doi.org/10.53975/lwol-8zok)
Abstract
Currently, antiviral drugs are targeted at specific viruses, requiring extensive research to develop de novo before a trial molecule is approved for a single viral target. However, it is possible that a strategic asset for mainstreaming antivirals has been hiding in plain sight. In our recent article published in Frontiers in Pharmacology, we explored the scope for plant-derived polyphenols, such as flavonoids, to be applied against infections spanning phylogenetically unrelated virus families. Beyond a long history of safe use in ordinary diet, common polyphenols also feature promiscuous binding behavior, a quality echoed by laboratory evidence that many are effective in vitro against viruses from diverse genera. Moreover, we mapped the research base undergirding the inflammatory response’s tendency to selectively return serum polyphenols from their latent, metabolized form back into their active, promiscuously-binding form uniquely at sites of inflammation -- such as those routinely established by viral infections. Verifying efficacy of flavonoids to constitute an annually-updated general antiviral protocol will require mobilizing a spectrum of animal model studies and clinical trials for initial confirmation.
December 17th, 2032:
During an exhausting red-eye flight across the country to visit with family over the winter holiday, you chide yourself for feeling angry over the passengers seated nearby you coughing up a storm. After all, they have no control over it and are wearing masks. You get off the plane, grab a coffee to go, and amble over to the terminal’s rideshare pickup area. A couple days later, you’re also coughing. You head to the pharmacy. They swab you and sequence whatever bug you got. It turns out to be this year’s strain of influenza-A virus — the flu. Your viral load was subsequently quantified from the same swab overnight; The results come back the next day: Just 10⁴, or 10,000, viral RNA copies per mL, nothing so strong (yet). The pharmacist asks if you’d like take-home treatment. Eager to avoid a week being laid up alone instead of time with the family, you gladly accept.
The pharmacist looks up which among the several hundred pre-cleared flavonoids (commonly-encountered compounds from plants) were most recently verified for effective antiviral activity against this year’s rhinovirus strain. This thanks to a newly expedited approval process ratified by the FDA’s board, where supplements with a long-familiar and highly tolerant human safety profile are verified for efficacy in animals and clinical trial volunteers who caught the same bug just months before you did.
She swabs you once more before handing you a bottle full of capsules of the relevant flavonoid, a pack of disposable vials of sample buffer, long cotton swabs, and a QR code for an app to download that will beep a reminder every four hours to take your next dose. Per the pharmacist’s instructions, you begin your flavonoid therapy while producing a fresh swab sample every day. By day 3, your symptoms no longer persist. You drop the samples off at the pharmacist who emails you a report the next day.
‘Your 2nd sample before starting the therapy was 10⁶ (one million) viral RNA copies per mL — a growing viral load. Your subsequent 3 home samples after commencing therapy clocked in at 10⁴ (ten thousand), 10³ (one thousand), and 10² (one hundred) copies per mL, consecutively. Your viral load is successfully receding to undetectable levels. Please continue your therapy to completion.’
To avoid experiencing the oft-reported viral rebound, you continue taking your flavonoid therapy for the rest of the week. Afterward you go on to enjoy a rejuvenating Christmas dinner with family.
Of course this scenario remains a distant fantasy; even as antibiotics are easily administered today against infections from a wide range of bacterial species, we still live in a world sorely lacking effective protocols against common virus species. Rare exceptions to this reality are rightfully celebrated as well-known feats of enormously hard-won scientific investment: Paxlovid for COVID-19, inhibitors for HIV and hepatitis-C spanning years of R&D, trials, and agency approvals. Could the fictional account describing an off-the-shelf general antiviral protocol ever become a reality? To answer that, we’ll need some context...
Got selectivity?
Whenever headlines pop announcing ‘researchers find molecule X inhibits the coronavirus’, contrarians will reflexively point to the famous 2013 “petri dish” comic from XKCD:
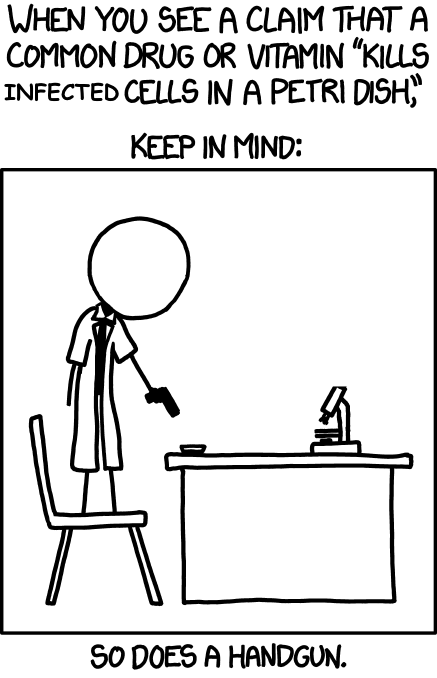
This cartoon vividly reminds us that not only must a compound make its way through the body’s metabolism to the inside of cells where it can disrupt the virus’ replicating, but it also must avoid disrupting healthy cells’ functioning. This property is called selectivity. The more selective a drug is, the more easily it is tolerated while still doing its job.
Flavonoids (no, not named for flavor...)
Before the pandemic, several research papers reported flavonoid inhibition of the SARS (from 2003) virus component called the main protease. In 2021, appreciating the structural similarity of the SARS protease to the pandemic’s SARS-CoV-2 protease, several stakeholders and I had set out to initiate a COVID-19 clinical trial leveraging a commonly consumed flavonoid derived from plants.
What is a flavonoid? Flavonoids are the most diverse of the three classes of natural compounds known as polyphenols. Flavonoids are produced in most plants and are widely acknowledged to serve an antimicrobial role among others. But over the past 30 years the plant biology literature has begun to recognize that flavonoids form a key part of plants’ defenses against plant viruses. We consume small quantities of different flavonoids every day when we eat fruits and vegetables. One tends to see the same flavonoids pop up in every paper researching them — diosmetin, hesperetin, quercetin, luteolin — yet there are a couple thousand flavonoid molecules catalogued.
In 2021, I led the drafting of our flavonoid clinical trial protocol for COVID-19. In the process I needed to elucidate a crucial lingering unknown: How large should the dose be? Scouring flavonoid pharmacology papers to answer that question yielded an under-reported but important finding. Many of the papers I dug up were making it clear that, evidently, there was an underreported selectivity mechanism in all the mammal species studied, humans included. If the mechanism was verified for the antiviral context, its value wouldn’t be restricted to just coronavirus work, but would apply to infections from many different families of viruses that met certain usual criteria. A more compelling research opportunity than just another COVID-19 therapeutic, with wider scope of future impact seemed to be presenting itself.
While there were a couple of reviews of this mechanism’s literature base in recent years, no one had yet revisited & updated it in light of the COVID-19 pandemic. Rather than add to the already substantial base of plant compound trial candidates for COVID-19, I thought it would contribute greater value if I did a deep-dive into fleshing out this putative selectivity mechanism. My hope then was to provide a more rigorous evidence base by painstakingly mapping out and illustrating how each of the relevant citations supported our understanding of the selectivity mechanism. With greater confidence and understanding of the selectivity property in hand, researchers would have a stronger foundation from which to investigate the antiviral properties of this broad class of well-tolerated plant compounds.
I brought my advisor in and we wrote a review manuscript in late 2021, which was recently published (October, 2022). So what’s it all about?
A sticky situation
Polyphenols have a reputation among medicinal chemists for being “sticky”. One med chemist even uses a more colorful metaphor to describe their interaction with proteins: “dog poop sticks to a blanket — any blanket”. My own longitudinal analysis of researchers’ bioassay contributions to the open public chemicals database PubChem demonstrates that polyphenols’ reputation for promiscuous binding is indeed well-earned. Polyphenols are easily found to bind to between 5%-30% of all protein/enzyme targets they are screened against. When you project that across the entire human proteome, that’s a whole lot of distinct protein & enzyme species.
This well-founded reputation for promiscuity however is then cited as a reason that polyphenols should be ignored from consideration as therapeutic leads for clinical investigation for any particular condition. Noted opinion-leader for industry med chemists, Derek Lowe, writes of the most frequently studied flavonoid, quercetin:
“It’s just that it [quercetin] shows up as a hit in so many assays, and has been linked to so many diseases and conditions. There are quite a few compounds in that category [polyphenols], and people have been burned by them in the past trying to get something to work...Is one compound good for all of those? The odds are very much against it, and when you see so many varied results, you start to wonder if it’s really much good for anything in particular.”
The question is, is that conclusion deserved? Are polyphenols really just promiscuous red herrings to be dismissed out of hand? To answer this question, we‘re going to look under the hood:
Polyphenols’ reputation for stickiness abounds because they contain multiple phenol structures (aromatic carbon rings with at least one hydroxyl group).

Hydroxyls are polar, exposing a hydrogen atom to form a hydrogen bond with electronegative atoms in protein/enzyme targets they happen to encounter. Hydrogen bonds are often employed in the med chemist’s toolbox to stick a ligand (a candidate drug molecule) to a target (an enzyme or protein of interest).

Just one example of what some med chemists think may be happening when aggregating molecules such as polyphenols bind promiscuously to enzymes — the enzymes essentially stick, like lint to a ball of yarn, onto these accumulated balls of polyphenols bound together:

That’s not to say that aggregation is definitely the promiscuous binding mechanism that a polyphenol could exhibit, but it is one such observed mechanism. Another, for example, could be a compound with many rotatable bonds that can easily conform to a protein target’s surface, sticking at relevant points-of-contact thanks to its several hydroxyl groups.
Promiscuous, but selectively so
A stockpile of promiscuous compounds leaves us in a precarious position evaluating polyphenols as antiviral candidates. Are they sticky and bind to everything indiscriminately? Or could they be sufficiently selective to avoid causing excessive trouble elsewhere in the body while hitting our intended targets of interest?
That takes us to the curious part. It turns out that, since the early 2000s, a widely dispersed band of researchers spanning Japan and Europe unexpectedly began to hit upon a mechanism that exhibited selectivity of all the polyphenolic molecules they investigated. The first researcher to strike upon this mechanism was Kayoko Shimoi, PhD at the University of Shizuoka in Japan. Like many natural product researchers, she was studying intestinal absorption of her polyphenol of choice, luteolin, in rats.
As is typical with other polyphenols, she verified that luteolin would see a glucuronic acid group added to it by the rat’s liver to prepare it for rapid elimination from the rat’s body. Pharmacologists generally recognize that once tagged with a glucuronide group, a molecule becomes so polar it becomes effectively impossible to passively enter cell membranes — a critical barrier to overcome for any would-be antiviral. This is because antiviral compounds must infiltrate through cellular membranes in order to act upon viruses replicating inside the cell.
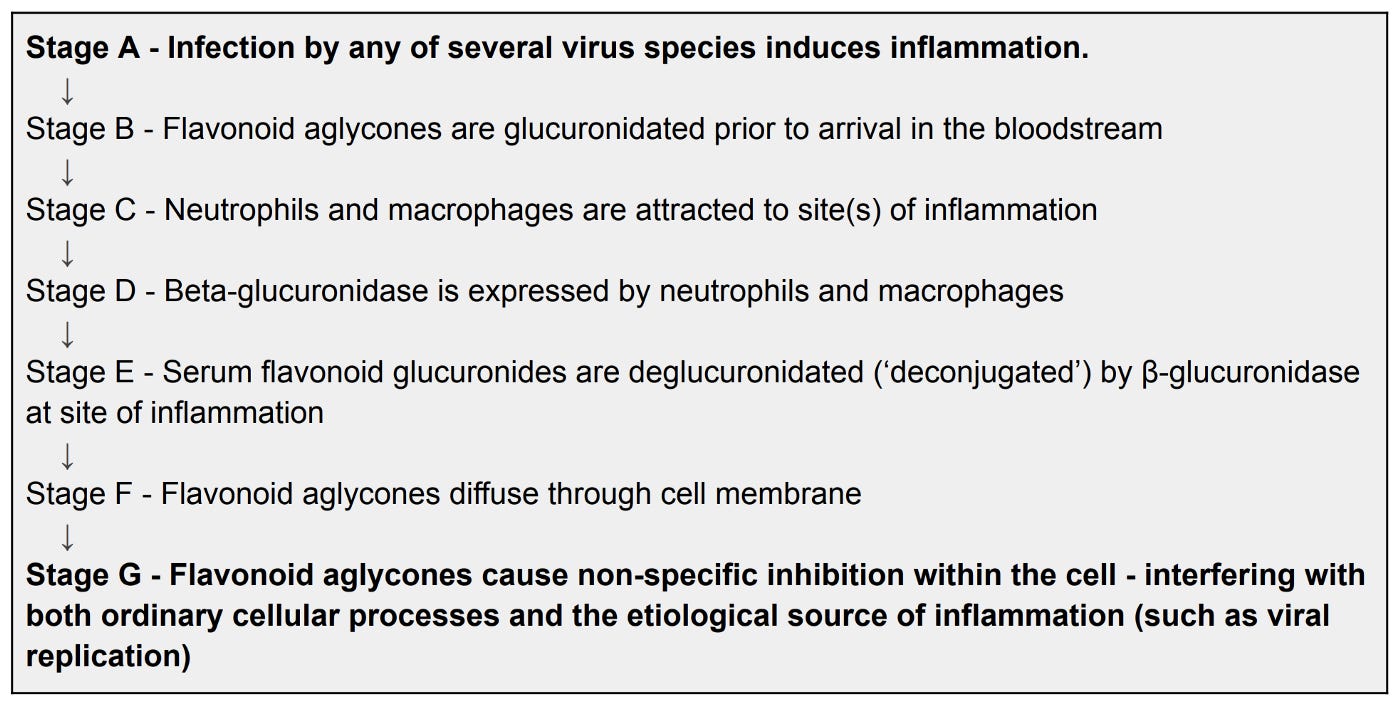
However if the rats were subjected to inflammation, like in the body’s natural immune response to having proteins from bacterial cell walls injected into them, the luteolin would lose the glucuronic acid component, reverting to its original & more potent, cell-penetrating form.
Taking the example studied by Dr. Shimoi of the conversion of the flavonoid luteolin → luteolin glucuronide...

...you can see the glucuronic acid in the top-left of the right-side image replacing one of the hydrogens from the left-side image (the luteolin core molecule, aka the aglycone).
Other researchers followed up Shimoi’s work by studying similar polyphenolic flavonoids like quercetin. All in all, we catalogued 20 papers (and several reviews) that cumulatively added support toward verifying that this deglucuronidation-through-inflammation mechanism is in fact a real phenomenon.
As later researchers would go on to uncover, the secret star of this show is an enzyme each of us produces called β-glucuronidase. Specifically, the identification that common white blood cells called neutrophils and macrophages accrete to sites of inflammation, and dump payloads of β-glucuronidase uniquely at those sites. The β-glucuronidase inevitably runs up against the glucuronidated flavonoid, ejects the glucuronic acid part, and voila, the flavonoid aglycone can suddenly passively enter cell membranes. Now it’s in a position to promiscuously inhibit many of the virus particles it encounters inside the cells. At the same time, the aglycone can just-as-promiscuously inhibit the infected cell’s ordinary enzymes, like those used for respiration and transcription. This is desirable considering the cell has already been co-opted by the virus to produce more virions. And because the serum flavonoid remains glucuronidated outside the inflamed site, it leaves the healthy cells happily intact. In other words, selectivity at its finest.
While the entire mechanism put-together formally looks like this:
From Sheridan, R. Spelman, K. (2022)
...it’s more easily comprehended graphically:

The graphic shows how quercetin loses its glucoside through the intestinal lining, going on to accept a glucuronic acid group before the liver releases it into the bloodstream. From there, in the case of a locally inflamed site such as from a viral infection, the glucuronide inevitably encounters the enzyme β-glucuronidase, losing its glucuronide and entering the infected cell’s membrane.
So perhaps there’s a mechanism — what can we do with it that we don’t know about already?
A shortlist of polyphenolic antiviral candidates
First and foremost, verification of the Shimoi selectivity mechanism reinforces the value of mining the extensive polyphenolic antiviral in vitro literature base to provide candidates for effective, well-tolerated antivirals. Researchers in polyphenolic antivirals are most likely correct in studying the aglycone in vitro as an antiviral rather than its glucuronide, so long as the virus under study induces the inflammatory response in vivo.
When researchers do antiviral screens in the lab with polyphenols (or with any other molecule for that matter), a critical step they inevitably undertake is to determine the IC50 (inhibitory concentration to reduce viral replication by 50%). Our survey of whole virus replication inhibition studies based on flavonoids yields this table of IC50 values:

And although there are many more studies that look at inhibiting the action of isolated components of a virus, such as a protease, polymerase, or spike protein, we disincluded them in this table. This is because we wanted to provide insight into the effects of all viral proteins & enzymes being inhibited by each study’s molecule, including any additional reduction in viral replication provided by inhibiting cellular processes that the virus had co-opted in infected cells.
In the laboratory, future researchers could expand this table to cover additional known viruses and viruses that emerge in future epidemics. In promising cases, they could go on to viral challenge studies in animal models (employing adequate biosafety protocols) & ultimately trials in humans who have caught the bug.
Therapeutic window
There’s another encouraging implication of Shimoi’s mechanism. While researchers produce IC50 values from laboratory antiviral screens, a critical step they also inevitably undertake is to determine the CC50 (the cytotoxic concentration, or cell-toxic dose that will kill 50% of healthy cells so administered) value. The higher that CC50 is a multiple of IC50, the better. 10X is a good starting point, and we would of course love to see 50X+ and beyond. That way, we can safely target trialing higher doses toward the IC90 or IC99 end of things (90% and 99% viral replication inhibition, respectively) without running headlong into the CC50.
This selectivity mechanism tells us that for polyphenol screens, the routinely determined CC50 value for the flavonoid aglycone is practically irrelevant to provide. Rather, the CC50 value of the glucuronide — not its corresponding aglycone — is more useful because that’s the form of the molecule that healthy cells will be exposed to. And happily, we have every reason to expect that the glucuronide’s CC50 value is much, much higher than the aglycone’s CC50, since the glucuronide can’t even pass through cell membranes into cells. This means we anticipate a much larger therapeutic window at the whole organism (animal & human) level to explore a therapeutic dose within than we would have otherwise inferred based on the aglycone CC50 alone.
Pharmacologically relevant polyphenol serum concentrations are challenging to achieve. Researchers are going to want to trial at high doses using animal models. The human equivalent dose of a couple thousand milligrams every four hours wouldn’t be an unexpected regimen, for example. A typical polyphenol’s time profile in serum resembles the below:
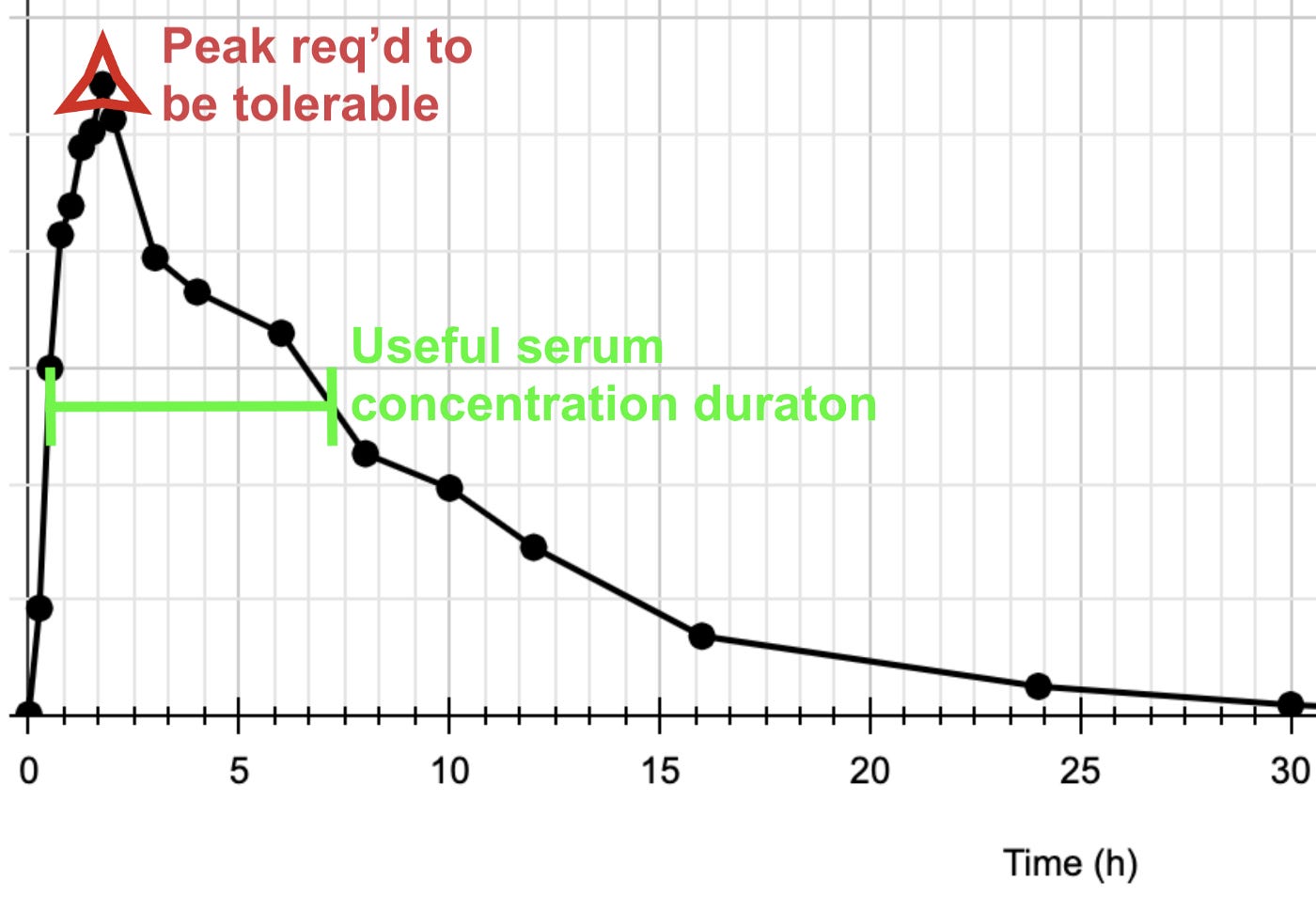
Note the sharp initial spike and fast decay. Clinicians will want a wide therapeutic window to work with so that the initial spike can be safely accommodated while maintaining a more prolonged IC90–99 concentration (at roughly the “shoulder” concentration in green below the spike) till it’s time for the next dose. A comfortably high glucuronide CC50 value, as indicated by the Shimoi mechanism, provides quantitative justification for more potent trial doses than those ordinarily administered in humans for other conditions (yet happily still far, far below TD50 toxicity values that are inferable from historical animal studies).
Evolutionary basis
We can only speculate on why the Shimoi mechanism exists. And to be sure, accepting its very existence should require further end-to-end verification in multiple animal species, humans included. Perhaps it is simply incidental that mammalian metabolism relates otherwise independent phenomena together.
Personally, I suspect a unified evolutionary basis. We mammals go back a long time, and our common ancestors who made it through evolutionary bottlenecks like the K-T extinction were small, rodent-sized creatures. Herbivores among them would unavoidably have been munching down therapeutic doses of polyphenols in the plants they ate. When lucky individual immune systems evolved the mechanism (strictly speaking, when their white blood cells began to express β-glucuronidase — all the other mechanism components were already present) they would have found themselves more robust to contemporary virus infections and natural selection would run its course. Maybe follow-on studies of the mechanism will ascertain that it’s not restricted to the Mammalia class, making it either much older or a case of convergent evolution.
What’s next?
Now focus turns toward building awareness for studies investigating therapies that exploit the putative Shimoi selectivity mechanism.
Facing would-be sponsors of any natural compound’s clinical trial is the challenge that natural products aren’t patentable in the US. In Europe, one can patent a natural compound for a particular illness if the results are verified, but then it’s a single-payer system for purchasing your offering — limiting upside. Since these are the markets pharma investors pay attention to the most, they will perceive a limited pay-off yet all of the financial risk & lead time that comes with running pharma studies & clinical trials. (If someone from the natural supplements industry would like to correct me, it would be welcome! :) ). Therefore my intuition is that it would have to be a philanthropic endeavor to support studies.
Suppose clinical trials repeatably show reduced viral load in animal and human clinical trials during a regimen with particular flavonoids. In that case, patients can reasonably be expected to become less sick — or even remain asymptomatic — until they clear their infection completely. The win at the end of it all would be a shortlist of well-tolerated plant compounds that can be indicated for viral infections emerging from a wide repertoire of unique virus species.
Then maybe the futuristic pharmacy scenario could one day become a reality.
In a nutshell:
There may be a class of compounds readily accessible from the plant kingdom that can inhibit viral replication (much as paxlovid does for SARS-CoV-2), with very generous safety profiles owing to a selectivity mechanism characterized over the past two decades. Verification of several polyphenols exploiting the mechanism to show safe antiviral effect in infections from diverse virus species would yield a strategic bank of antiviral compounds. Public health officials could draw from this stockpile facing future pandemics. During an emergent outbreak of a novel virus, so long as it induces an inflammatory response in human hosts, then the strategic bank of polyphenols could be quickly assayed and clinically trialed to determine which among them is most effective for reducing the new virus’ viral load in infected patients. And yes, after annual assays & verifications against seasonal nuisance viral illnesses, the local pharmacy could stock them as well.
Disclaimer
This piece advocates for supporting a particular line of clinical research and nothing more. Emphatically, no one should read this and consider self-administering discussed compounds for the purposes described. More than just boilerplate, there are relevant compounds and corner cases I’ve not covered here that my conscience would really, sincerely prefer readers not do trial-and-error on.
Acknowledgements
The author would like to thank everyone acknowledged in the manuscript, stakeholders & supporters of last year’s proposed clinical trial, M. Hu PhD for technical consultation, and D. Stein, S. Molnar PhD, Dr. Angela Reiersen MD-PhD, S. Ferguson PhD, and K. Spelman PhD for
reviewing article drafts & encouraging feedback. The author recognizes the OpenAI initiative for tool support during composition of the abstract. Finally, the author would like to give special recognition to Alexandra Elbakyan without whose tireless efforts to make scientific research available would have rendered the present work impossible to conceive.
Gardener Comments
Josh Randall:
This was a well-written, accessible review of a topic in need of additional nuance in the public sphere. The description of the selectivity mechanism was useful for a reader to develop a mental model of the immune components and polyphenols. It also allows for there to be room to explain the huge variety of proteins known to interact with flavonoids while explaining the apparent immune benefits that occur with viruses. Focusing on this area and the connections this has to society and our 'sci-fi' conception of solving viral infections was very successful, but the short inclusion of a hypothesized evolutionary source and financing mechanisms seemed unnecessary. If the author would plan to include the evolutionary hypothesis, evidence of specifically shared genes, gene families, or regulatory mechanisms with other species with this system could be supportive.
Scott Ferguson:
I found it to be a very intriguing hypothesis (re: inflammation / macrophage / polyphenol pharmacology axis) and really well compiled! It’s very compelling and well-written. I think it’s easy to understand/ accessible. One point I’m not so sure of is the fraction of glucuronidated compound that is excreted into the blood for urinary excretion (and would therefore be systemically available) and what fraction is excreted into bile (this is the mechanism of b-glucuronidase I am familiar with but is a enterohepatic loop, so minimal systemic availability). For the fraction that is systemically available the next hurdle in my mind is pharmacokinetics, if the inflammation site is hard to diffuse to (i.e. nasal passage) it may be more quickly eliminated in urine than it can appreciably accumulate at the relevant site to be deglucuronidated and available to act. All said, it’s a very interesting mechanism that inflammation generally can cause local expression of b-glucuronidase, which in the context of evolution and flavonoids in our foods seems an obvious mechanism of combating diseases, like viral infections. I think for sure it highlights the importance of eating healthy. It certainly warrants being studied more closely too if high doses of certain flavonoids can be even more potent.
Angela M. Reiersen, MD, MPE:
It would be wonderful to have a list of naturally-occurring polyphenols (or other natural substances) that would each be effective in treating multiple types of viral infections. This could potentially make immediate treatment of acute viral illnesses safe, easy, and accessible for people throughout most of the world. If some of these substances are effective against multiple viruses, this could be a huge advantage in case of future pandemics. As I read this article, I was thinking what a shame it is that there are so many barriers to researching safety and efficacy of so-called natural products.
Most drug companies would prefer to develop and test new drugs rather than repurpose existing off-patent drugs, let alone develop products containing only natural, often unpatentable, substances. Also, I think many physicians tend to be wary of claims that natural products have health benefits. These concerns are often for good reason: We hear too many natural product proponents claiming that natural supplements must be better than manufactured drugs, simply because they are "natural", ignoring the fact that organisms can naturally produce toxic substances as well as beneficial ones.
There is evidence that many naturally occurring, biologically-created substances are beneficial--or even essential--for good health, and in some cases there is even specific evidence of antiviral effects (at least anecdotally or in the laboratory environment), but in most cases there have not been enough clinical trials in humans to be sure of any benefit against COVID-19 illness.
The COVID-19 pandemic made it very important to test existing manufactured drugs and naturally-occurring products in randomized clinical trials as quickly as possible, to increase the chance of finding effective treatments during the time when they were most needed. Yet, many of the COVID-19 treatment trials preregistered--on clinicaltrials.gov or elsewhere--were never completed. It seems there were not adequate systems and resources in place to immediately fund, organize, and implement a large number of these trials.
At Washington University, Eric Lenze and I were able to quickly start up a clinical trial of the repurposed drug fluvoxamine for treatment of COVID-19 at the start of the pandemic (Lenze et al, 2020), which has now shown evidence of benefit in treating COVID-19 in trials that used relatively high doses (Lee et al., 2022), and less evidence when a lower dose was used (Bramante, 2022). We could not have accomplished our initial fluvoxamine trial without recognition by our institution's COVID research committee that the work was important (this allowed us to go ahead and apply for ethical approval), and then a speedy review by our institutional review board, allowing us to begin the trial while we still had a high number of COVID-19 cases in our area.
I have colleagues at some other institutions who made efforts to conduct trials of existing drugs and/or natural products but ran into hurdles such as lack of funding or lack of approval by hospitals, academic institutions, or governmental agencies. I think there were a lot of missed opportunities to potentially find treatments that could have helped reduce severe COVID-19 illness, hospitalizations, and deaths. It is impossible to know how many lives might have been saved if more high-quality COVID-19 early treatment clinical trials had been done early in the pandemic, but it is not too late to improve the situation now.
To minimize the damage from further SARS-CoV-2 infections, especially by new variants against which current vaccines and pharmaceuticals may be less effective, more clinical trials are needed. Repurposed drugs like fluvoxamine and naturally occurring substances such as polyphenols may act on host factors and/or have nonspecific effects against multiple types of viruses, so may be less likely to lose their benefit as new genetic variants arise. While newly developed drugs can be helpful, they are likely to be expensive and take time to develop. Once produced, they are typically not immediately available worldwide. If there are inexpensive repurposed drugs and natural substances that can reduce morbidity and mortality related to COVID-19 and other viral illnesses, showing this through clinical trials is of urgent importance. It is also critical at this time to focus efforts on trials of potential long COVID treatments. Some people who had COVID-19 early on, in the first wave of the pandemic, have been dealing with substantial long COVID symptoms for about 3 years at this point. They have waited far too long for clinical trials and proven treatment options. If multiple natural products turn out to be beneficial for long COVID, that would be wonderful, especially if some are widely available as components of common plants, fungi, or other organisms in many areas of the world. If multiple natural substances turn out to be helpful, it would be much easier for people to extract various effective substances from locally occurring organisms than to wait for new drugs to be manufactured and distributed across the world.
I hope this work with polyphenols and other naturally-occurring substances leads to improved treatment for many viral illnesses, and that well-proven treatments will be made available and affordable to everyone across the globe.
References:
Bramante CT, Huling JD, Tignanelli CJ, Buse JB, Liebovitz DM, Nicklas JM, Cohen K, Puskarich MA, Belani HK, Proper JL, Siegel LK, Klatt NR, Odde DJ, Luke DG, Anderson B, Karger AB, Ingraham NE, Hartman KM, Rao V, Hagen AA, Patel B, Fenno SL, Avula N, Reddy NV, Erickson SM, Lindberg S, Fricton R, Lee S, Zaman A, Saveraid HG, Tordsen WJ, Pullen MF, Biros M, Sherwood NE, Thompson JL, Boulware DR, Murray TA; COVID-OUT Trial Team. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. N Engl J Med. 2022 Aug 18;387(7):599-610. doi: 10.1056/NEJMoa2201662.
Lee TC, Vigod S, Bortolussi-Courval É, Hanula R, Boulware DR, Lenze EJ, Reiersen AM, McDonald EG. Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022 Apr 1;5(4):e226269. doi: 10.1001/jamanetworkopen.2022.6269.
Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, Miller JP, Yang L, Yingling M, Avidan MS, Reiersen AM. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA. 2020 Dec 8;324(22):2292-2300. doi: 10.1001/jama.2020.22760.
Chen, Yu Wai:
This article presents an interesting peek into one possible scenario of future personalized medicine.
Inspired by a drug development exercise during the COVID-19, the author extended his studies and highlighted that flavonoids may be exploited as general therapeutics that are selectively acting against many pathogenic agents—any that causes an inflammation, including but not limited to viruses.
It is worthy of noting that flavonoids can be universal binders also because of the planar aromatic ring that is hydrophobic. Flavonoids turn up frequently in computational drug screening investigations presumably because of their convenient sizes and adaptable shapes, owing to the rotatable bonds connecting the rings.
The author discussed that flavonoids are harmless in normal body state, yet their access to cells is enhanced at the sites of inflammation. Once these molecules enter the cell, they can inhibit many enzymes, viral and native body enzymes alike. The author argued that this is an advantage because infected human cells are already hijacked by the viruses. However, this may be an issue when the inflammation is at non-renewable cells such as neurons.
Nevertheless, this idea is worthy of further actions and research.
Overall, I think this article is well written and is comprehensive to the general readers, although the necessary chemistry may still deter some. This hypertexted article contains links to additional information (webpages, Wikipedia, academic journal articles) when more elaborated explanations of important concepts are introduced.
Conflict of interest: The reviewer knows the author personally. We have no formal collaboration in research. Our relationship is one that is between scientists and our occasional exchange of information mainly regards sharing of drug developments news of COVID-19.
Dan James:
This paper is written in an accessible opinion-piece style that presents a compelling case for the potentially highly beneficial use of flavonoids, a class of plant-derived compounds able to treat viral infections. The writing style is confident, assured and obviously written by an expert or experts in the field.
The paper makes a convincing case for the therapeutic advantages of flavonoids and the need for further research. After the experience of the COVID pandemic, it would seem an obvious matter of urgency that all possible means to inhibit viral replication are explored, especially if some candidate compounds are readily accessible from the plant kingdom.
However, this is where the reason for the paper’s advocating style becomes apparent - the problems of patenting natural products and the financial risks involved in clinical trials with long lead times.
These problems of financing research and trials lead the author/s to conclude that it will take a philanthropic intervention to bring about the potential and very desirable outcome of well-tolerated plant-derived compounds able to treat a range of viral infections. If true, this is a serious indictment of the ‘free’ market capacity to supply the public good of health and an unfortunate demonstration of how knowledge production can be negatively determined by economics.
Coincidentally whilst reviewing this paper, I also came across some articles on the challenges of introducing psychedelic drugs as potential treatments for some mental health conditions. There are parallels between flavonoids and psychedelics – both originally plant-derived and both ‘hard sells’ to conventional pharmaceutical companies or investors, so it strikes me there are possible synergies between the two areas.
The development of psychedelic drug treatments has benefited enormously from visionary investors like Christian Angermayer, who, with his company Atai Life Sciences, has vigorously pursued a policy of patenting psychedelics and has the view that - “Pharma is just a very boring distribution machine, they’re never willing to take any risks. We took the risk early and they all want to partner with us now’. Hopefully, for the sake of a healthier future for all of us, a similar visionary can be found for flavonoids.
This paper is an important one that deserves widespread dissemination, so I have no hesitation in recommending it for publication.
Jack Arcalon:
Very readable overview of a possible new paradigm of antiviral "antibiotics" that seems worthy of extensive investigation. Might also have many other effects on hidden chronic health problems that lead to more serious conditions of many types.
Dr. Payal B. Joshi:
The paper is written in an intriguing manner and the topic is relevant in today's time resonating with our quest for antivirals. I believe the article is presented in an insightful manner. Of particular bits that I instantly loved about the article are 1) Shimoi’s mechanism and 2) calling polyphenols "stick" (yes, indeed). I highly recommend the publication of this article.
Ted Wade:
Maybe it’s not that simple, but it sounds like a promising lead.
Appendix
This chart on the next page shows how the evidence base is supported by the literature by graphically mapping out the citations in the Shimoi mechanism literature base, showing precisely which of the four legs of the hypothesis, (C, D, E, F, or combinations of these like CDE), each citation supports.
Rick Sheridan leads EMSKE Phytochem fostering collaboration for plant-derived antivirals research. He also runs an agriculture industry venture in East Africa.
EMSKE Phytochem builds awareness and fosters collaboration for plant-derived antivirals research. Its work has been published in Towards Data Science, An Idea, and Frontiers in Pharmacology.
Donations: You can support Rick’s work by buying him a coffee.